Blog
Structure of pre-fusion state of the HIV Env trimer determined
Before HIV can enter a cell, the so-called Envelope spike on the viral surface needs to bind receptors on the target cell. This binding, researchers believe, causes the spike to open up and expose a normally hidden, inner portion of Envelope called gp41 that drives fusion of the viral membrane with that of the target cell, allowing the virus to enter. The structure of this opened “pre-fusion” state of the Env spike is unknown, but of great interest to researchers because it could inform the development of vaccine immunogens that induce antibodies that prevent the subsequent fusion of the membranes.
Now, a research team led by Sriram Subramaniam at the National Cancer Institute in Bethesda has determined the three-dimensional shape of the pre-fusion state (PLoS Pathog. 8, e1002797, 2012). Subramaniam and his colleagues used an approach called cryo electron microscopy (cryo EM), which involves freezing a protein very quickly in liquid nitrogen to preserve its natural structure, and then taking thousands of EM images of the protein from different angles and merging the images to reconstruct a high-resolution three-dimensional image. “This state has never been seen before,” says Subramaniam.
To create this so-called “activated intermediate” state of the Env spike, the team took the part of the Envelope trimer protein that lies outside the viral membrane and mixed it with an antibody called 17b, which they had found to be able to bind and open the spike. To keep the spike from opening too far, and to stabilize it in the pre-fusion state, the researchers used a previously engineered Env protein that has a disulfide bond that connects the inner gp41 portion with the outer gp120 portion of Env, which are normally only held together by weak, non-covalent forces.
They then froze the Env trimers that had 17b bound to them, and determined the three-dimensional structure of the complex with cryo EM at a resolution of nine Angstrom, the highest resolution ever reported for the structure of trimeric HIV Env. They found that in the activated intermediate state of the Env trimer, the helices at one end of the gp41 protein were exposed, consistent with an existing model that proposes that the virus inserts these helices into the target cell membrane to fuse it with the HIV membrane (see figure below). “We have an extremely exciting, vulnerable state of HIV where [the spike] has just been opened but has not yet gone to [the] post-fusion state,” Subramaniam says. “If you attack it at this stage, then potentially you could catch it before anything happens.”
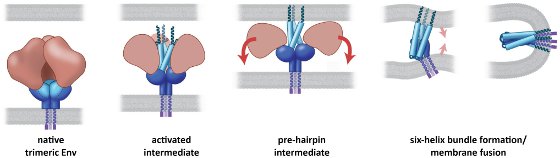
| Model for the Mechanism of Env Activation |
|
Binding of the native trimeric Env spike to the CD4- or co-receptor (not shown) on the target cell triggers formation of an activated intermediate, in which the outer gp120 part of the Env trimer (red) opens and exposes three helices at the N-terminal end of gp41 (blue). In the pre-hairpin intermediate, the ends of these helices insert into the target cell membrane (gray stripe on top), and the outer gp120 part of the Env trimer (red) is believed to fall off. Finally, the association of the three N-terminal gp41 helices to form a six-helix bundle causes the target cell membrane and viral membrane to approach each other and fuse. Image courtesy of Sriram Subramaniam, National Cancer Institute. The image originally appeared in PLoS Pathog. 8, e1002797, 2012. |
Indeed, Subramaniam and his colleagues are now planning to synthesize molecules that mimic the activated intermediate for use as potential vaccine immunogens that might induce antibodies that inhibit membrane fusion. The exposed parts of the gp41 helices on the activated intermediate are the most conserved portions of HIV, Subramaniam says, which means that it would be difficult for HIV to develop escape mutations from such antibodies.
“[It’s] absolutely fantastic to see an activated intermediate with clear definition of gp41 helices,” says Peter Kwong, a structural biologist at the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases, who was not connected to the study. “It will be fascinating to see if these helices are the target for Fuzeon (enfuvirtide), the only FDA-approved entry inhibitor."
The findings also suggest that researchers may have to rethink their assumption that many HIV strains need to bind to both the CD4 receptor and the CCR5 co-receptor to enter cells. Subramaniam’s team found that the 17b antibody, which binds the same spot on the Env spike as the CCR5 co-receptor, could bind and open the Env spike in the absence of CD4, suggesting that CCR5 alone might be able to trigger the formation of the open Env conformation, allowing HIV to enter a target cell. “It’s a new way of thinking about how entry happens,” Subramaniam says. “We can not prove yet that co-receptors do the same, but this is a plausible, although provocative hypothesis.”
In another set of experiments, Subramaniam and colleagues mixed HIV particles with two broadly neutralizing antibodies (bNAbs), b12 and VRC01, and used cryo EM to study if and how the binding of the bNAbs changes the structure of the Env spike. They found that VRC01 binding didn’t require any shape changes of the spike, whereas b12 could only bind after the Env spike partially opened. Subramaniam says this difference could explain why b12 neutralizes fewer HIV strains than VRC01, even though both bNAbs bind the same CD4 binding site on the spike: Not all HIV strains have an Env that can open to allow b12 to bind and neutralize them.
The cryo EM analysis also showed that VRC01 binding locked the Env spike in the closed state, making it impossible for the virus to enter the target cell. This might explain why VRC01 is such a potent antibody against HIV. “[VRC01] can recognize [the spike] as is and lock any further changes,” Subramaniam says.