Blog
Researchers Persevere in their Efforts to Understand HIV Persistence
The many facets of HIV cure research were discussed and debated this week in Boston at the Keystone-sponsored symposium Mechanisms of HIV Persistence: Implications for a Cure. As the name suggests, one of the main topics on the agenda here was how researchers can gain a handle on understanding the dormant pools of HIV-infected cells that are collectively referred to as the reservoir.
This reservoir of infected cells is established quickly after initial infection and can come roaring back if an individual interrupts antiretroviral therapy, which effectively suppresses ongoing viral replication. Take for example the case of a US infant who was presumed cured—the HIV-infected “Mississippi baby” started antiretroviral therapy soon after birth but when therapy was stopped more than two years later, the virus returned. Cases like this illustrate just how difficult finding a cure will be and how much of an obstacle HIV persistence really is.
The meeting in Boston, which kicked off with a rousing and optimistic call from Nobel laureate and AIDS co-discoverer Francoise Barré-Sinoussi, focused on efforts to develop latency and persistence models to allow researchers to zero in on where the virus lies dormant; the role that CD4+ T cells expressing the protein receptor CTLA-4 play in the ability of HIV to persist; the activity of ‘natural killer’ cells in the unique VISCONTI cohort of 14 HIV-infected individuals who are in remission after stopping antiretroviral treatment; and the tools for both measuring latent reservoirs and different strategies for coaxing these cells out of latency so they can be targeted by antiretrovirals or antibodies and ultimately destroyed. “There’s just so many different stories,” says Steve Deeks, a co-organizer of the conference from the University of California in San Francisco.
Other highlights of the week included a talk by Robert Siliciano of Johns Hopkins University, who discussed potential ways to measure the efficacy of therapeutic interventions; an ongoing debate over whether, in advanced disease, replication-competent virus can be found in the large white blood cells called macrophages; and discussions about the possibility that the central nervous system is another place where HIV lies dormant.
Guido Silvestri from Emory University, another co-organizer, focused on three questions that seem to be plaguing virtually all cure efforts: what are the mechanisms responsible for the establishment and maintenance of the viral reservoir; how can researchers best measure and monitor this reservoir; and what are the most promising approaches for eliminating it. One strategy for eliminating the reservoir is using some agent to roust HIV from dormancy and then use a combination strategy (likely antiretrovirals combined with the broadly neutralizing antibodies that are also fueling vaccine discovery efforts these days) to kill the virus as it awakens—the so-called “shock and kill” approach.
What to use to reactivate latent virus is an area of ongoing research, but data from a compound developed by Gilead Sciences (a toll-like receptor 7 agonist in development for Hepatitis B and C infection; see PrEP Works) is attracting considerable interest.
Ron Germain, chief of systems and lymphocyte biology at the National Institute of Allergy and Infectious Diseases, is developing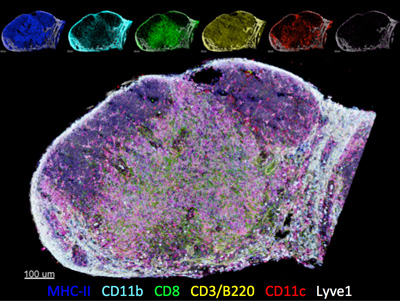 ways to visualize and eventually quantify the HIV reservoir. The point of the highly resolved imaging (see image, right), Germain says, is to try to detect integrated virus or viral RNA to help identify cells that are productively- and latently-infected either in nonhuman primates infected with simian immunodeficiency virus (SIV; the monkey form of HIV), or in HIV-infected people before, during, or after antiretroviral treatment. The idea is that if researchers can actually see where the cells are, they can phenotype and quantify them. Emerging data, however, show the team still needs to develop ways to assess an infected cell’s capacity for viable virus production. “It’s not a simple task,” says Germain.
ways to visualize and eventually quantify the HIV reservoir. The point of the highly resolved imaging (see image, right), Germain says, is to try to detect integrated virus or viral RNA to help identify cells that are productively- and latently-infected either in nonhuman primates infected with simian immunodeficiency virus (SIV; the monkey form of HIV), or in HIV-infected people before, during, or after antiretroviral treatment. The idea is that if researchers can actually see where the cells are, they can phenotype and quantify them. Emerging data, however, show the team still needs to develop ways to assess an infected cell’s capacity for viable virus production. “It’s not a simple task,” says Germain.
Meanwhile, Afar Okoye, a member of Louis Picker’s lab at the Oregon Health & Science University, shared early results from SIV-infected macaques showing that early treatment with antiretrovirals limits the seeding of the viral reservoir. “We can see that what happens during the acute phase of infection determines a lot when it comes to how much the reservoir is present and how much it lasts,” Okoye said. In this study 65 monkeys were given a low-dose intravenous injection of SIVmac239 and started on therapy at various time points from as early as four to five days after infection to 42 days after.
“Delay of even a couple days of therapy has a huge impact, you can see that,” Deeks says about Okoye’s findings. “If you’re going to have an impact on the reservoir, there’s no time to waste. You have to get in there and you have to start therapy immediately. Waiting a day or two can have a lifelong impact on the reservoir. The data is not subtle. The effect is dramatic.” Most clinics tell people who want HIV tests to wait until at least six weeks after potential exposure. But in San Francisco now, Deeks says if clinicians think people are infected they start them on antiretroviral therapy immediately. The introduction of faster tests and even home testing kits, as recently became available in the UK, may spur this effort.
The energetic Deeks is focusing part of his efforts on activating latent HIV reservoirs so they can then be wiped out. To this end, Deeks and his lab are examining Antabuse, or disulfiram, an old-line enzyme inhibitor discovered by accident in a rubber factory and used to help alcoholics stop drinking. “The Siliciano laboratory found it had a modest effect on reversing [HIV] latency,” Deeks says. “Not nearly as powerful as some of the other drugs being looked at. But it has a well-known safety profile and can be taken indefinitely.” This is a tortoise-and-hare story for him. “You can go in big with TLR-7,” he says, risking more potential toxicity, “or you can take your time and do it slowly.”
While cure research is certainly gaining momentum and prominence, it is still in the early days. Much too early, according to some researchers, to be tested in clinical trials that involve HIV-infected volunteers interrupting the antiretroviral treatment that is keeping their virus in check. David Margolis, a microbiologist from the University of North Carolina, says one one such trial is currently being discussed by the AIDS Clinical Trials Group. “There is a study proposed in humans to measure a bunch of things, using various markers, and then interrupt therapy and try to associate time to rebound,” Margolis says. He’s not so keen on the idea. “Why now?” he says. “We can hardly measure anything. Let’s do the work. Let’s take the time. What’s the rush?” Deeks, who would be part of the team to execute the planned study, concedes the point. “We’re not curing people, so why stop therapy?” he says. “The counter-argument is that interruptions can be done safely if people are monitored carefully, and we are just never going to advance the field unless we stop therapy because we don’t really have the capacity to measure the virus in blood. We don’t have a biomarker that people believe to be legitimate.”
For that, the search continues. –Michael Dumiak


