Blog
Antibodies Re-Energize Vaccine Investigations
Ever since Edward Jenner developed the first successful vaccine against the dreaded smallpox virus, researchers have been riveted by the interplay between the body’s defense mechanisms and pathogens staging an attack.
In a comprehensive review article published recently in Nature Immunology, two leaders in the field of HIV vaccine research discuss in detail the different types of antibody responses and advances in understanding both how these antibodies work to block HIV and how researchers are using this information to d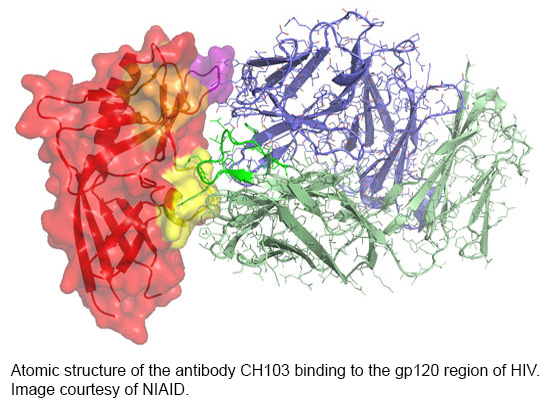 evelop vaccine candidates. John Mascola, director of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID), and Dennis Burton, director of the International AIDS Vaccine Initiative’s (IAVI) Neutralizing Antibody Consortium at The Scripps Research Institute (TSRI) in La Jolla, California, write in particular about broadly neutralizing antibodies (bNAbs) that can inactivate or neutralize multiple strains of HIV.
evelop vaccine candidates. John Mascola, director of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID), and Dennis Burton, director of the International AIDS Vaccine Initiative’s (IAVI) Neutralizing Antibody Consortium at The Scripps Research Institute (TSRI) in La Jolla, California, write in particular about broadly neutralizing antibodies (bNAbs) that can inactivate or neutralize multiple strains of HIV.
Discoveries related to bNAbs are propelling the field forward at a rapid pace. In 2009, scientists from several collaborating institutions isolated two bNAbs from a sample donated by a person participating in an observational study led by IAVI. These antibodies were much more potent and better able to neutralize HIV than the handful of neutralizing antibodies that were identified previously. Then in 2013 scientists at Weill Cornell Medical College in New York and TSRI were able to obtain an image of the principal target for bNAbs—HIV’s outer surface protein, the envelope trimer—greatly aiding vaccine researchers scouting out points of vulnerability on the virus. Mascola and Burton write that: “…the broadly neutralizing [antibodies] suggest that a vaccine against HIV-1 should be possible, if researchers can meet the challenge of designing immunogens to elicit such [antibodies].”
In their paper, Mascola and Burton describe three classes of antibodies. One antibody group fails to neutralize viruses but possesses other anti-viral capabilities. The second type neutralizes highly specific strains of virus. Scientists are most focused on the third group, the bNAbs, which take years to evolve in the body and exhibit qualities strikingly different from the other kinds of antibodies. Scientists like Mascola and Burton are actively involved in studying bNAbs. Several labs have already characterized antibodies that can broadly neutralize HIV. In an approach known as retro-vaccinology, scientists are engaged in working back from those antibodies to identify immunogens (the components of vaccines that induce an immune response) that can elicit them.
No one is suggesting that a vaccine is just around the corner. Momentum is building, however, and not just in the vaccine realm. These bNAbs are also being pursued as an HIV treatment strategy or even as a component of an eventual cure. Scientists gathered in Maryland June 3-4 for an invitation-only meeting titled “Development of Monoclonal Antibodies for HIV Treatment and Cure.” NIAID and the Bill & Melinda Gates Foundation sponsored the meeting. Discussion points included the design of clinical trials to evaluate the use of bNAbs for treatment and cure research.
“It’s significant that they are acting in a more coordinated way at this stage of research,” said Richard Jefferys, the Basic Science, Vaccines, and Cure Project coordinator for the Treatment Action Group, an advocacy organization in New York City. “There’s enough encouraging data to say that studies in people need to be done.”
- Kitta MacPherson is a writer for VAX and IAVI Report in New York and an award-winning science journalist


