IAVI Report readers might find this paper released by the Global Health Technologies Coalition interesting. It looks at the challenges faced by organizations working on solutions for diseases associated with poverty. The authors find that product development partnerships—a model for addressing public health needs that was recently covered in IAVI Report— play a central role in bringing together resources and the expertise of different sectors involved in related R&D. Yet there remain critical gaps in such efforts, the study finds--many associated with funding, regulatory processes, and the limited capacity for research and manufacturing in developing countries. The paper also looks at possible solutions to the identified obstacles.
Blog

Uncategorized
- 25 posts in this category
A functionally cured CROI baby? Check out our latest issue of VAX for more details of this interesting case, which was presented at the 20th Conference on Retroviruses and Opportunistic Infections. The March VAXalso covers therapeutic vaccination and the start of a new AIDS vaccine trial in Africa that employs a novel viral vector.
John Mellors is the chief of infectious diseases at the University of Pittsburgh. His research focuses on antiretrovirals (ARVs), such as HIV resistance to them, using them for preexposure prophylaxis (PrEP) to prevent HIV infection in individuals, or modeling to see how PrEP affects the HIV epidemic. He also studies HIV reservoirs in patients on antiretroviral therapy (ART) and how to eliminate them. Mellors is also a member of the scientific advisory board of Gilead Sciences, the maker of Truvada, a combination of the ARVs tenofovir and emtricitabine. On May 10, Mellors spoke on behalf of Gilead at a hearing, where an expert advisory panel recommended to the FDA to approve Truvada for PrEP. The FDA is expected to make a decision in September. If approved, this would make Truvada the first ARV to be approved for PrEP. At the recent Cold Spring Harbor meeting on retroviruses, Mellors gave a Keynote address on how to eradicate HIV from the individual and from the world, focusing on cure research and antiretroviral drugs. I had the chance to talk to him about recent progress in cure research, and about what FDA approval of Truvada for PrEP would mean.
Compared with children in developed countries, children in the developing world show a poorer response to oral vaccines (such as oral polio vaccine), whereas their response to vaccines that are administered systemically by injection (such as measles vaccine) doesn’t appear to be much different.
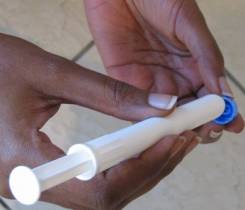
You need not look further than the headlines today for the biggest news to emerge from the International AIDS Conference in Vienna. For the first time, a microbicide candidate was shown to reduce the risk of sexual transmission of HIV in women. The candidate, a 1% gel formulation of the antiretroviral tenofovir, reduced the risk of HIV infection by 39% in a South African trial known as CAPRISA 004, which involved 889 women.


